Everything You Need to Know About Sodium hydroxide (NaOH) — A Complete, Engaging Guide

If you’ve ever seen “lye,” “caustic soda,” or “soda lye,” you’ve encountered sodium hydroxide. This powerful chemical is used in everything from soap-making to industrial water treatment. In this article we’ll cover what sodium hydroxide is, its formula, whether it’s a base or acid, its pH, hazards, structure, molecular weight, melting/boiling points, solubility, common names and symbols, uses (12 major ones), and safety. Let’s dive in, and I promise to keep it friendly, clear and even fun for a chemistry fan.
What is Sodium Hydroxide?
Sodium hydroxide is an inorganic chemical compound composed of sodium (Na⁺) and hydroxide (OH⁻) ions. It is popularly known as “caustic soda” or “lye.”
It is industrially produced in large quantities (tens of millions of tonnes globally) because its strong alkaline nature makes it extremely useful in many processes. Wikipedia+1
Sodium Hydroxide Formula
The chemical formula for sodium hydroxide is NaOH: one sodium (Na) atom, one oxygen (O) atom and one hydrogen (H) forming the hydroxide ion.
Common Names and Symbol
Common names: caustic soda, lye, soda lye. Wikipedia+1
Symbol: NaOH (chemical symbol/formula)
Synonyms: sodium hydrate, sodium hydroxide (solid).
Is NaOH a Base or an Acid?
Good question! The answer: NaOH is a strong base.
Because it dissociates in water to give hydroxide ions (OH⁻) which raise the pH (make it alkaline). For example, the hydroxide ion reacts with H⁺ (protons) to form water, thereby reducing acidity and increasing alkalinity. InChem+1
So if you see “NaOH solution,” think strong alkali. It neutralises acids rather than behaving as an acid.
Physical and Chemical Properties
Here are the core physical/chemical properties of sodium hydroxide to know — we’ll go through molecular weight, structure, melting & boiling points, solubility etc.
Molecular Weight
The molecular (molar) mass of NaOH is ~ 40.0 g/mol (often quoted as 39.997 g/mol).
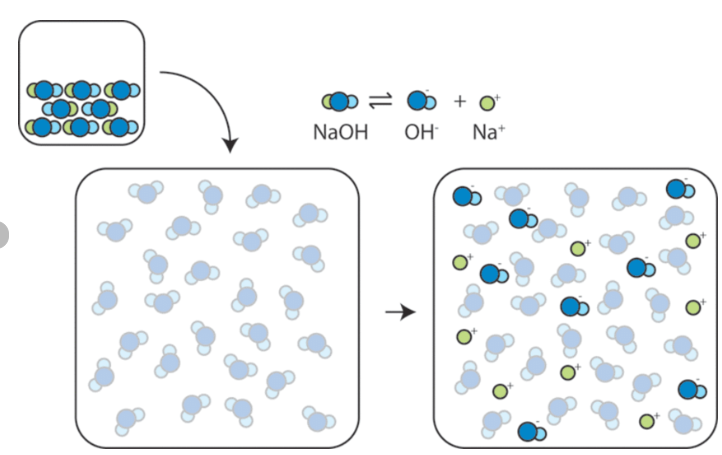
Structure
In the solid crystalline form, sodium hydroxide consists of sodium cations (Na⁺) and hydroxide anions (OH⁻) arranged in a lattice.
When dissolved in water, it dissociates fully into Na⁺ and OH⁻ ions.
Visually, you can imagine Na⁺ and OH⁻ ions in a lattice or in solution as separated ions.
Melting Point and Boiling Point
- Melting point (solid → liquid) of NaOH: about 318 °C.
- Boiling point (liquid → gas) of NaOH: around 1,388 °C (or sometimes ~1,390 °C in some sources).
Solubility
NaOH is very soluble in water. For example, at ~20 °C, one source reports ~ 109 g per 100 mL of water.
It is also soluble to a lesser extent in polar solvents like ethanol or methanol (but not in non‐polar solvents). ChemicalBook
pH of Sodium Hydroxide
When you make a solution of NaOH in water, because it produces OH⁻, the pH is very high (alkaline). For example, a 0.5% solution has a pH around ~13. ChemicalBook+1
In practice, you’ll commonly see NaOH solutions with pH in the 12-14 range depending on concentration.
Hazards of Sodium Hydroxide
Because of its strong alkaline nature and ability to generate heat on dissolution or reaction, sodium hydroxide must be handled with care. Below are the key hazard points.
Corrosivity and Health Hazards
- NaOH is highly corrosive to skin, eyes, mucous membranes and tissues. Contact with solid or concentrated solution can cause severe chemical burns and permanent damage. Wikipedia
- Inhalation of dust, mist or aerosols can irritate or damage the respiratory tract, potentially leading to swelling of the airway, inflammation, or pulmonary edema.
- It is odorless, so there may be no warning smell in vapour/dust form.
Reactivity Hazards
- NaOH reacts violently with acids (neutralisation gives heat) and with certain metals (like aluminium, tin, lead, zinc) producing hydrogen gas, which is flammable/explosive.
- When solid NaOH contacts water or moisture, the dissolution is highly exothermic — heat can cause splattering, burns or ignite combustible materials. Wikipedia
Environmental Hazards
- Releases of NaOH to water/soil can raise pH and harm aquatic organisms. It is corrosive to metals and can damage infrastructure.
Safe Handling Tips
Always add NaOH to water (not water to NaOH) to avoid violent boiling/heat release.
Wear appropriate personal protective equipment (PPE): chemical‐resistant gloves, eye/face protection, protective clothing, ensure good ventilation.
Store in air‐tight, moisture‐resistant containers, away from acids, metals, organic materials and moisture/humidity.
In case of exposure: flush skin/eyes with large amounts of running water for a prolonged period and seek medical attention.
Uses of Sodium Hydroxide — 12 Key Applications
odium hydroxide is extremely versatile. Here are 12 important uses, with some explanation of why it’s used.
- Soap manufacturing & detergents – NaOH is the main alkali used in “cold-process” soap making (for hard bar soaps). The hydroxide ion reacts with fats/triglycerides (saponification) to form soap + glycerol.
- Drain/oven cleaning – Its strong alkalinity dissolves grease, oils, hair and other organic clogging materials, turning them into more water‐soluble products.
- Pulp & paper (kraft process) – In the paper industry, NaOH is used to separate lignin from cellulose in wood, assisting in making pulp and recycled paper.
- Textile industry – Used in processing cotton fabrics (mercerisation), manufacturing artificial fibres (e.g., rayon), and in bleaching and dyeing.
- Water treatment / pH adjustment – It is used to raise the pH of water (e.g., in drinking water treatment or industrial effluents), neutralise acids and reduce corrosion in piping systems.
- Food industry – Specifically regulated uses include: curing foods (e.g., olives), peeling fruits/vegetables (tomatoes, potatoes), pH adjustment in food processing.
- Aluminium refining – In the extraction of alumina (from bauxite) using the Bayer process, NaOH dissolves the alumina component leaving impurities behind.
- Chemical manufacture / pH control – NaOH is used in many chemical syntheses as a base, in neutralisation steps, and in making sodium salts.
- Metal cleaning & electro-plating – Used to clean metal surfaces, remove oxides, prepare surfaces for electroplating or coating.
- Oil refining – Used in petroleum industry to remove acidic components (organic acids or sulphur compounds) from crude oil and improve quality of fuels.
- Recycling & waste treatment – In paper recycling to remove ink from fibres; in waste treatment for alkaline neutralisation and metal recovery.
- Cosmetics & pharmaceuticals – At low concentrations, NaOH is used as a pH adjuster in creams, lotions, hair relaxers (professionally used). Note: only in very controlled amounts and neutralised later.
These uses make sodium hydroxide a foundational industrial chemical, often taken for granted but absolutely essential.
sodium hydroxide: Detailed FAQ
Here we answer the “People Also Ask” style questions you listed.
What is NaOH called?
NaOH is called sodium hydroxide. It is also commonly called caustic soda, lye, soda lye or sodium hydrate.
What are the hazards of sodium hydroxide solution?
- Concentrated sodium hydroxide solution is highly corrosive and can cause severe burns to skin, eyes, mucous membranes, throat and gastrointestinal tract if ingested.
- Inhalation of mist/dust may irritate and damage the respiratory system, possibly causing swelling or pulmonary damage.
- The dissolution of solid NaOH in water or reaction with acids is highly exothermic (releases heat) and may cause splashing or ignition of flammable materials nearby.
- Reacts with certain metals producing hydrogen gas (flammable).
What is sodium hydroxide used for?
(See the 12 uses listed above.) It’s used in soap & detergent manufacture, drain cleaning, pulp & paper, textiles, water treatment, food processing, aluminium refining, chemical production, metal cleaning, oil refining, recycling & waste treatment, and cosmetics/pharma.
What is the pH of sodium hydroxide?
A solution of sodium hydroxide is strongly alkaline (high pH). For example, a 0.5% solution of NaOH has a pH around 13. ChemicalBook
The exact pH depends on concentration: higher concentration = higher pH (closer to ~14).
Summary Table of Key Data
| Property | Value / Description |
|---|---|
| Formula | NaOH |
| Molecular (molar) mass | ~ 40.0 g/mol |
| Physical appearance | White crystalline solid, hygroscopic (absorbs moisture) |
| Density | ~2.13 g/cm³ |
| Melting point | ~318 °C (solid) |
| Boiling point | ~1,388 °C |
| Solubility in water | Very high (e.g., ~109 g/100 mL at ~20 °C) |
| pH (in solution) | Very high (strongly alkaline) – e.g., pH ~13 for 0.5% solution. |
| Common names & synonyms | Caustic soda, lye, sodium hydrate, soda lye |
| Major uses | Soap/detergents, drain cleaning, pulp & paper, textiles, water treatment, food processing, aluminium refining, chemical manufacture, metal cleaning, oil refining, recycling, cosmetics/pharma |
| Main hazards | Corrosive to skin/eyes/respiratory tract; exothermic when dissolving; reacts with metals to produce hydrogen gas; environmental risks |
Tips for Further Reading / Safe DIY Use (some friendly guidelines)
If you ever DIY (yes: note “DIY” as a keyword) with sodium hydroxide (for example, in soap-making), always respect safety: use proper gloves, goggles, work in a well-ventilated area, add NaOH to water (not vice versa), and ensure the mixture has cooled before handling.
In DIY soap-making, although NaOH is corrosive, once reacted (saponified) it is no longer caustic in the final bar (if your fat/oil + NaOH reaction is complete). But the early stages demand caution.
For industrial or home uses (cleaning, drain clearing), be aware of concentration: household drain cleaners with high % NaOH are still dangerous.
If you work in an environment (lab, factory, water treatment) where NaOH is used, ensure you know the MSDS/SDS (safety data sheet) for the grade you handle.
When disposing of solutions with NaOH, avoid releasing highly concentrated alkaline pH into waterways/soil; neutralise where required and ensure environmental compliance.
Final Thoughts
Sodium hydroxide (NaOH) may look like a simple chemical formula, but behind it lies:
- A strong base, one of the workhorses of chemistry and industry.
- A compound with very high pH, so strongly alkaline it can burn skin and damage materials.
- A material with impressive physical properties (very high melting/boiling points, high solubility, hygroscopic nature).
- A substance with wide use across soap, detergent, paper, textile, water treatment, food, metal, oil, recycling, cosmetics and more (12 major uses we listed).
- A hazardous chemical that demands respect – from handling solid pellets or concentrated solutions to mixing it safely and storing appropriately.
- A “chemistry story” that touches our daily lives in subtle but significant ways — making it a compelling subject for readers who want to see how chemistry truly matters in the real world.